..
Tissue manufacturing systems present unique challenges to biocontrol efforts. The use of recycled fiber and presence of reducing agents often lead to performance upsets due to the negative impact of these factors on the efficacy of antimicrobial agents used to control contamination. These challenges are further complicated by variability in the concentration of reducing agents present and the degree of microbial loading into the process.
Many systems utilize the minimum amount of chemical required to control microbial growth to reduce costs and chemical consumption. A lack of real-time monitoring tools has limited the ability to proactively respond to process variability to prevent production upsets. An on-line monitor has been developed to allow for real-time, continuous monitoring of microbial activity and surface deposition without the need for reagents. Proactive monitoring ensures continuous optimization of treatment programs and more efficient use of antimicrobial agents.
This new approach has allowed many mills to improve machine efficiency for extended operation between boil-outs. This reduces demand for water, boil-out chemicals, and energy required to heat incoming water to process temperatures for an improved environmental footprint. Case studies are presented which demonstrate the ability of the on-line, proactive optimization strategy to improve sustainability of tissue manufacturing.
By Laura Rice, Senior Research Scientist, Tobias Schild, Industry Technical Consultant, Charlie Foss, Industry Technical Consultant, Nalco, An Ecolab Company
INTRODUCTION
Tissue manufacturing systems provide ideal conditions for microbial growth. If biocontrol is inadequate, microbial growth can lead to spoilage of fiber stock and additives resulting in offensive odors in the final product. Microbial growth can also lead to the formation of surface deposits, which may contain microorganisms as well as chemical components, including wood pitch, stickies, wood fiber, and other wet end additives. The light weight of the end product makes tissue and towel grades especially sensitive to the build-up and sloughing of surface deposits, which may lead to holes and breaks. In an effort to manage microbial growth in a cost effective manner, tissue makers are relying to a greater extent on oxidant-based biocontrol programs.1
A significant percentage of tissue and towel is manufactured using a high content of recycled fiber from a variety of sources. Recycled fiber, such as recovered newsprint, mixed office waste, and old cardboard containers serves as an excellent substrate for microbial growth during collection, storage, and processing. The microbiota in recycled fiber is highly diverse, including several microbes that are known to contribute to spoilage and slime formation in additives and/or the process system (Figure 1).2,3 Remarkably, it has been reported that the mean biocide cost per ton for tissue or towel manufactured using recycled fiber was 188% above the mean cost for virgin tissue or towel.4
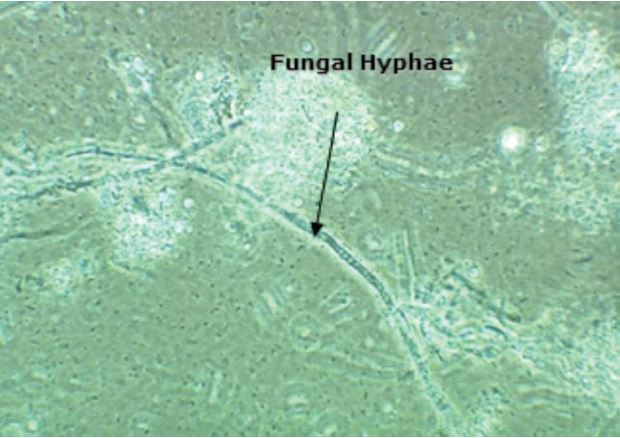 Figure 1 – Tissue machine deposit indicating the presence of bacteria and fungi, along with chemical material.
Figure 1 – Tissue machine deposit indicating the presence of bacteria and fungi, along with chemical material.
The processing of recycled fiber can also introduce chemical reducing agents (e.g. sulfite). Fiber carrying sulfite residuals into the wet-end places exceptional stress on oxidant-based biocontrol programs. Microorganisms, metabolic byproducts of microbial growth, and chemical reducing agents create demand for oxidant-based control agents. This leads to biocontrol performance upsets due to the negative impact of these factors on oxidant persistence and efficacy. These challenges are further complicated by variability in the concentration of reducing agents present and the degree of microbial loading into the process. Many systems utilize the minimum amount of chemical required to control microbial growth to reduce costs and chemical consumption; therefore, it is extremely difficult to ensure adequate treatment in response to variable conditions in oxidant demand. Recovering from a contamination event requires much higher treatment levels compared to preventing process contamination, particularly if microorganisms form surface deposits.5
Traditional monitoring approaches (e.g., plate counts and adenosine triphosphate, ATP) may not analyze process water samples frequently enough to detect changes that render the biocontrol program ineffective. Furthermore, traditional microbiological monitoring techniques are labor intensive, and reagents can be costly. These approaches may allow for diagnosis of problems related to microbial growth; however, a lack of real-time monitoring tools hinders the ability to proactively respond to process variability to prevent production upsets.
An on-line monitor was developed to allow for real-time, continuous monitoring of microbial activity and surface deposition. The real-time approach allows for rapid detection of increased bacterial loading and oxidant demand, so the impact of these factors on the oxidant-based biocontrol program can be minimized (Figure 2). Proactive monitoring ensures continuous optimization of treatment programs and more efficient use of antimicrobial agents.
OxiPRO® MONITOR
The OxiPRO Monitor is a patented instrument that provides real-time evaluation of process conditions, microbial metabolic activity, and surface deposit formation to allow for proactive modification of the treatment strategy in response to process variability.6 Process water is passed through the monitoring device and data are collected continuously. This is a reagent-free device, which requires minimal maintenance. The side stream of process water that passes through the monitor is returned to the process unchanged. Respiration by planktonic and sessile organisms in the sample flow cell is measured under carefully controlled conditions and is used to determine the level of activity in the process (Activity Index). Oxidation-reduction potential (ORP) is measured as an indicator of adequate oxidant dosing and control of microbial growth. Surface fouling can also be monitored through optical analysis of a translucent disc that is exposed to process water.7
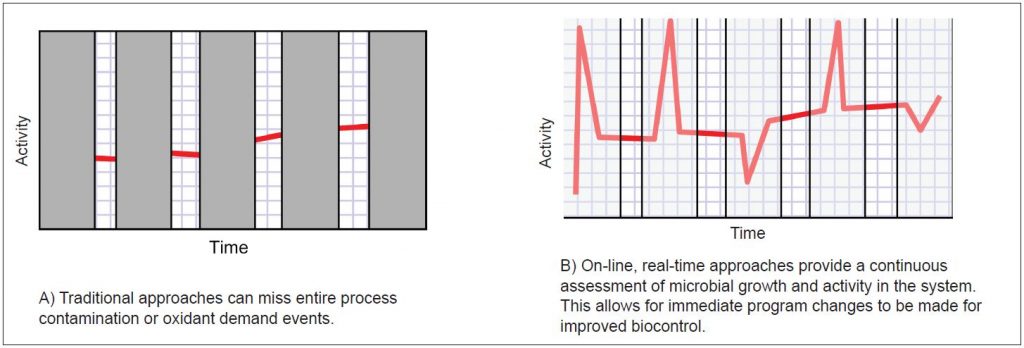 Figure 2 – Traditional batch testing approaches (e.g. standard plating and adenosine triphosphate (ATP)) do not detect variability, while real-time monitoring detects variability allowing for pro-active treatment to prevent biocontrol upsets.
Figure 2 – Traditional batch testing approaches (e.g. standard plating and adenosine triphosphate (ATP)) do not detect variability, while real-time monitoring detects variability allowing for pro-active treatment to prevent biocontrol upsets.
NALCO PROACTIVE TREATMENT STRATEGY
On-line monitoring ensures treatment levels are adequate in response to changes in process conditions that may consume oxidant-based biocontrol programs at different rates. Data can be interpreted rapidly to allow for pro-active control of microbial growth for prevention of spoilage and deposit formation that can reduce production efficiency and impact product quality (Figure 3). Data collected from the OxiPRO Monitor is representative of conditions in the process water and on machine surfaces (Figure 4).
Due to the variability of conditions in tissue and towel manufacturing processes, one treatment strategy is not effective in all processes for an indefinite period of time. On-going optimization is required to maintain machine efficiency. In tissue and towel grades, the high use of recycled fiber and resulting sulfite residuals leads to high oxidant demand. Therefore, it may not be effective to treat the process continuously with an oxidant-based treatment strategy because dosages would frequently be inadequate. While a slug treatment strategy might be cost-effective by allowing the oxidant to overcome the demand to provide an effective oxidant residual, if dosing is too infrequent or the slug concentration is too low contamination events will result.
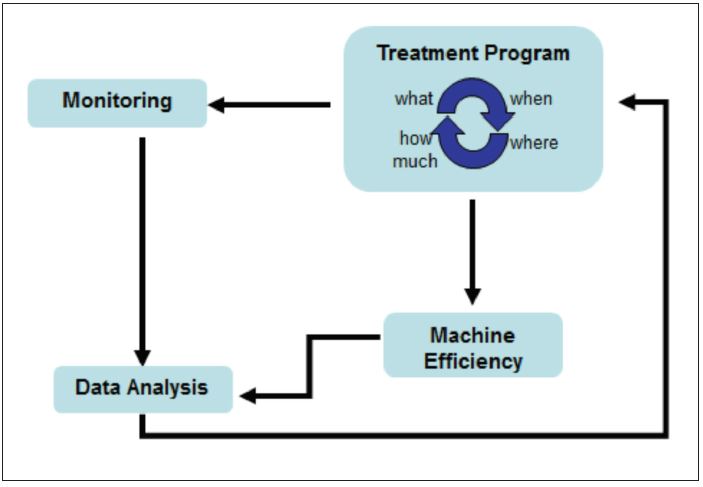 Figure 3 – On-line microbial monitoring allows for proactive optimization of biocontrol programs to maintain machine performance and product quality.
Figure 3 – On-line microbial monitoring allows for proactive optimization of biocontrol programs to maintain machine performance and product quality.
While a slug feed strategy for oxidant-based biocontrol programs can be effective in systems with high oxidant demand, it is critical to ensure that effective concentrations are achieved at appropriate frequency. Slugs that are too frequent may not provide adequate control due to efforts to minimize chemical
consumption, which results in inadequate concentrations of oxidant at the peak of the slug event. Furthermore, simply measuring on-line ORP does not provide adequate information regarding oxidant-based biocontrol program performance. These measurements may indicate that dosing has achieved a target ORP value. However, this ORP target may not correlate with adequate control of microbial growth and deposit formation. There is a need to combine ORP with on-line activity to understand the impact of the oxidant-based biocontrol program and ORP targets on microbial growth in real-time. Figure 5 demonstrates that similar treatment levels result in varying degrees of microbial control depending on the frequency and concentration of the oxidant slug.
 Figure 4 – Conditions and microbial activity measured in the OxiPRO Monitor are representative of conditions which exist in the process system. This information can be used for pro-active optimization of biocontrol programs.
Figure 4 – Conditions and microbial activity measured in the OxiPRO Monitor are representative of conditions which exist in the process system. This information can be used for pro-active optimization of biocontrol programs.
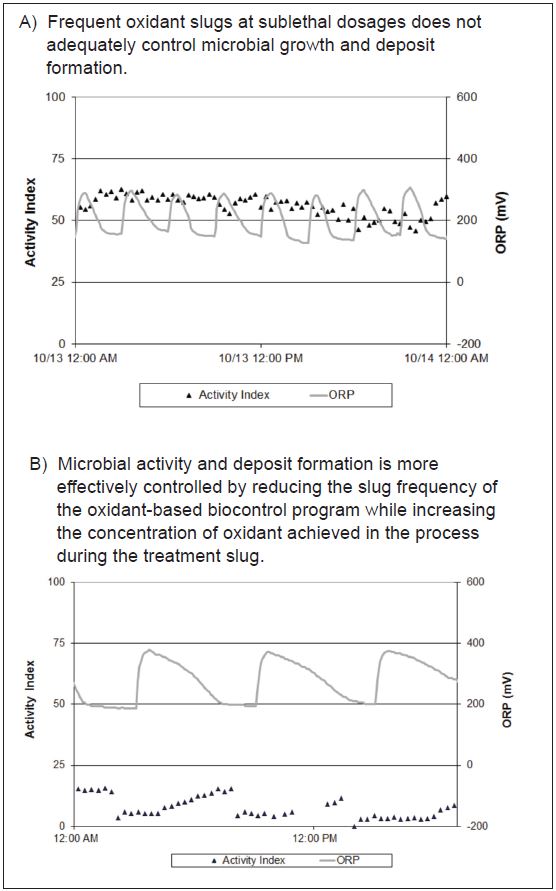 Figure 5 – Slug dosing frequency, duration, and concentration of oxidant-based biocontrol programs can be monitored using ORP values and the impact on microbial activity can also be measured using the OxiPRO Monitor to ensure optimum biocontrol performance.
Figure 5 – Slug dosing frequency, duration, and concentration of oxidant-based biocontrol programs can be monitored using ORP values and the impact on microbial activity can also be measured using the OxiPRO Monitor to ensure optimum biocontrol performance.
A CASE STUDY
A mill targeting production of hygienic tissue from 100% deinked pulp from recycled fibers was required to meet internal product specifications. Surface contamination could not exceed 1,000 colony forming units per milliliter and pathogens were required to be below the detection limit. An oxidant-based microbial treatment program was dosed continuously to various process locations in an attempt to guarantee the above mentioned specifications.
Biocontrol upsets occurred frequently due to process variability. Nalco implemented an OxiPRO biocontrol program utilizing a stabilized-oxidant program in combination with the on-line OxiPRO Monitor.
The OxiPRO program was implemented using a slug-feeding strategy to overcome the high demand and microbial loading entering the system with the recycled fibers. The ability to maintain higher oxidant concentrations during and after the slugs significantly improved microbial control in the system. Figure 6 highlights the reduction in microbial activity after the implementation of the new, integrated program.
Furthermore, the on-line monitoring strategy allowed for the detection of increasing microbial activity following the start-up of the tissue machine after an extended shutdown. The utilization of spoiled stock led to increased oxidant demand in the system, which overwhelmed to oxidant-based biocontrol program.
Prior to the implementation of OxiPRO integrated treatment strategy, an upset of this magnitude would have resulted in end-product quality that did not meet specifications. Using the monitor as a pre-warning tool the chemical dosage was increased and microbial activity returned to an acceptable level. This ensured continuous production of first-class, quality tissue.
The implementation of the OxiPRO integrated treatment strategy also significantly improved the overall cleanliness of the machine environment. Figure 7 shows the wirepit running a stabilized-oxidant program (A) and after the implementation of the OxiPRO integrated treatment strategy (B).
CONCLUSIONS
• The on-line OxiPRO Monitor assesses the impact of process conditions on the presence of oxidant-based biocontrol agents (ORP) and performance of biocontrol program in real-time (Activity Index).
• Proactive monitoring ensures continuous optimization of treatment programs allowing for the ability to meet product quality specifications, improved machine performance, and extended operation between boil-outs.
• Proactive monitoring and treatment allows for more efficient use of antimicrobial agents and reduced demand for water, boil-out chemicals, and energy required to heat incoming water to process temperatures for an improved environmental footprint.
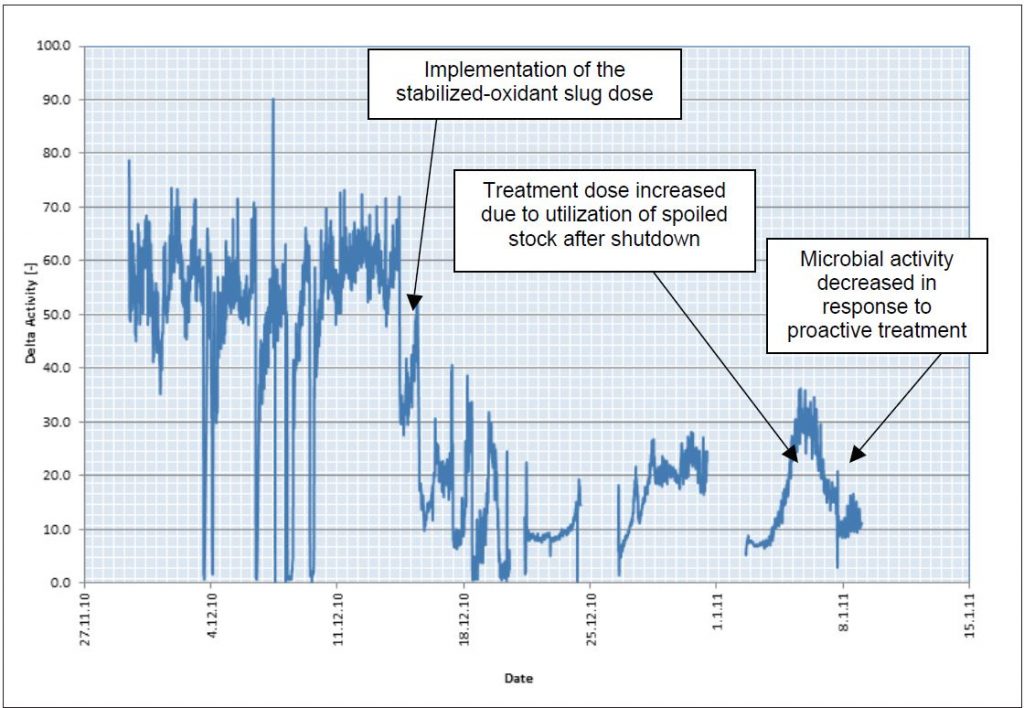 Figure 6 – The OxiPRO Monitor documents microbial activity before and after the implementation of the
Figure 6 – The OxiPRO Monitor documents microbial activity before and after the implementation of the
integrated biocontrol program.
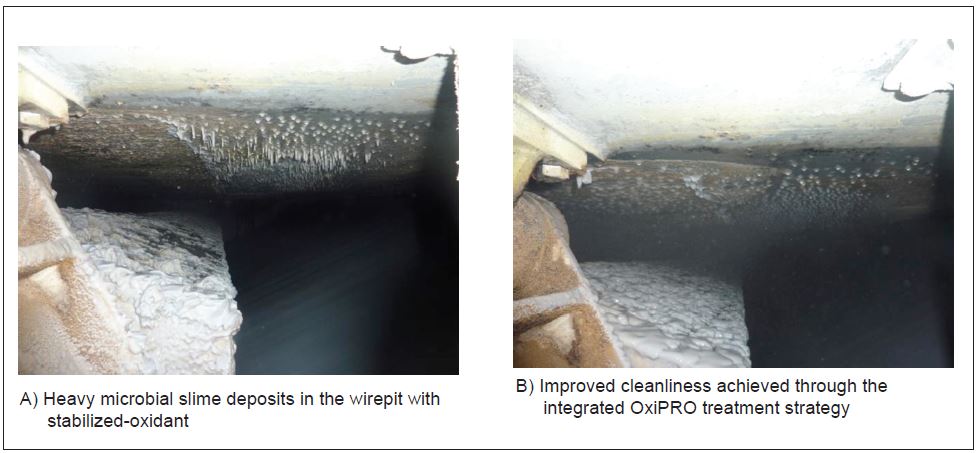 Figure 7 – Comparison of the wirepit cleanliness using different treatment strategies.
Figure 7 – Comparison of the wirepit cleanliness using different treatment strategies.
REFERENCES
1. Foss, C., “Advanced Microbiological Monitoring and Control with New Stabilized Oxidant Programs,” Tissue World Americas 2008 Conference Proceedings, Miami Beach, Florida.
2. Suihko, M.-L., and E. Skytta. 2009. Characteristics of aerobically grown non-spore-forming bacteria from paper mill pulps containing recycled fibers. J. Ind. Microbiol. Biotechnol. 36:53-64.
3. Suihko, M.-L., and E. Skytta. 1997. A study of the microflora of some recycled fibre pulps, boards, and kitchen rolls. J. Appl. Microbiol. 83:199-207.
4. Sorelle, P.H., and W.E. Belgard. “The effect of recycled fiber use on paper machine biological control,” 1991 Papermakers Conference Proceedings.
5. Costerton, J.W., S. Lewandowski, D.E. Caldwell, D.R. Korber, H.M. Lappin-Scott. 1995. Microbial Biofilms. Annu. Rev. Microbiol. 49:711-745.
6. Enzien, M. V., Rice, L. E., Ashton, S.B. Method of Monitoring Microbiological Activity in Process Streams. US Patent 8,012,758. 6 September 2011.
7. Wetegrove, R.L., and R.H. Banks. Monitoring of Film-formers. US Patent 5,155,555. 13 October 1992.




Comments are closed.