…
Retention aids are commonly used in tissue manufacturing to retain fines and get improvements in machine efficiency and costs of operation. And, although tissue makers generally know that wet-end chemistry can impact dry-end creping performance, there is often little understanding of the typically positive effects these materials have on creping. In addition, there are very few technical papers examining the cause and effect relationships of these phenomena.
In this paper we examine the positive impacts that retention aids can have on Yankee coating properties and creping performance. Mechanistic explanations are provided to help understand the improvements in the creping operation that are often observed. Case studies demonstrate how these positive effects – which may include increased coating on the dryer, improved wet-end stability and improved felt cleanliness – can be utilized to improve product quality and further reduce costs of operation.
By G. Furman, R. Phillips and S. Archer, NALCO Water – An Ecolab Company
A. INTRODUCTION AND BACKGROUND
1. The basics of Retention on Tissue and Towel Machines
Retention of fiber, fines and additives is sometimes referred to as the key to efficient papermaking. This is no less true in tissue and towel furnishes than it is on fine paper machines. The appropriate use of retention aids can improve machine and additive efficiencies, as well as contribute to improved sheet properties. Although retention of fillers is not typically a concern in tissue and towel, the retention of fines and strength additives are still important considerations.
Retention of particles in the fiber web will be greatly influenced by three properties – size, surface area and surface charge. A challenge of retention in any papermaking system is the wide range of particle sizes present in a given furnish. This size range can span five orders of magnitude from the relatively large fibers (1-3 mm) to very small colloidal materials (0.01-3µ). Fiber fines are classically defined as those fiber fragments able to pass through a 76 µ (200 mesh) screen.1 The surface area of the particles is another important consideration since the fiber fines can have approximately 10 times greater surface area than the fibers. The larger the surface area the greater the demand will be for a particular additive, such as a retention aid, or wet strength agent. Finally, the electrical charge of the surface of the particle is important in the mechanism of retaining it in the fiber web.
Retention mechanisms can be classified into three basic types as listed below:
1. Mechanical Entrapment
2. Chemically Assisted Retention
3. Combination of (1) and (2) above.
Mechanical entrapment of furnish components will depend on the relative ‘openness’ of the forming fabric and the pore size range of the fiber web as it consolidates during sheet formation. Fiber retention will occur by mechanical entrapment. Chemically-assisted retention is necessary for retention of the much smaller colloids, as these materials are too small to be mechanically entrapped. Retention of fines can occur through a combination of chemical and mechanical means. Chemical agglomeration can increase small particles to a size range where they can now be mechanically retained.
Chemically assisted retention can be further discussed in terms of the mechanisms shown in Table 1.2,3 These mechanisms will result in the attachment of colloidal and fines materials to the larger fibers and/or an increase in the effective particle size so that the materials are retained in the sheet. Coagulation of particles can be described as a charge neutralization process that is determined by the balance between electrical double layer repulsion and van der Waals attraction forces. Particle surface charges are neutralized and or screened, thus compressing the thickness of the electrical double layer. The repulsive energy barrier between the particles is reduced, the attractive forces take over, and the particles agglomerate as shown in Figure 1. Table 1 – Chemically Assisted Retention
Table 1 – Chemically Assisted Retention
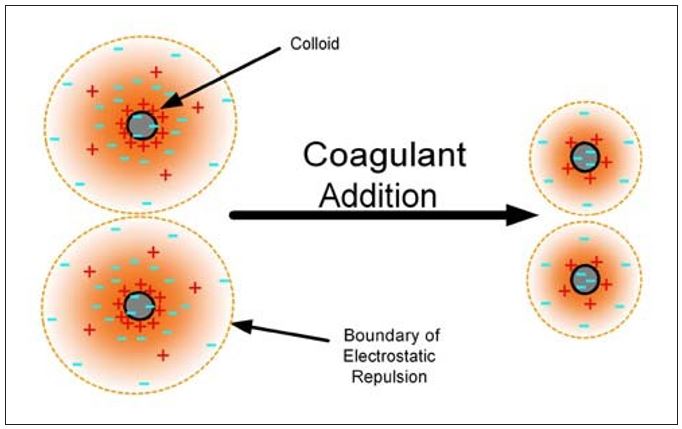 Figure 1 – Compression of the repulsive double layer by a coagulant allows particle agglomeration.
Figure 1 – Compression of the repulsive double layer by a coagulant allows particle agglomeration.
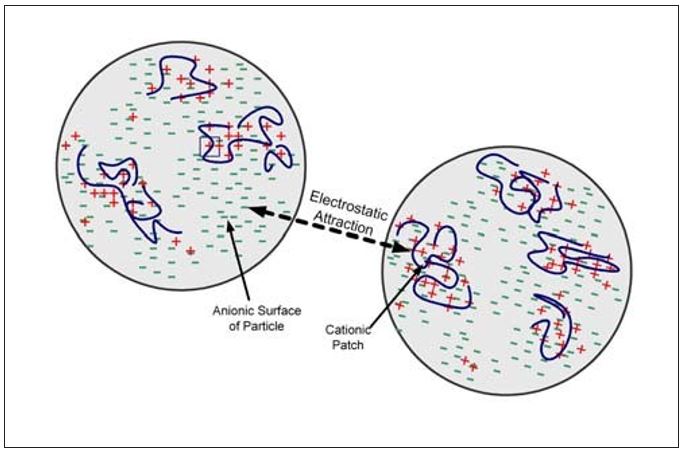
Patch flocculation is descriptive of the flocculation mechanism where a polymer adsorbs onto a particle surface forming a cationic patch. As shown in Figure 2, cationic and anionic surfaces then exist on the same particle. Flocculation between particles occurs due to electrostatic attraction between two oppositely charged surfaces. The degree of attraction depends on the charge density of the cationic polymer and the degree of surface coverage. Complete coverage and neutralization is not needed for patch flocculation to take place and, in fact, would cause re-dispersion of the particles. Polymers that are good patch flocculants are characterized by short to moderate molecular weights and high charge densities. They must be able to extend outside the electrical double layer of the particle to be effective. The flocs that are formed tend to be small and compact. These flocs can also reform after shear-induced deflocculation. That is, the flocculation is reversible.
Bridging flocculation is illustrated in Figure 3. In this mechanism, the desired adsorbed polymer conformation on the particle is one having long loops and tails. This polymer conformation is influenced by both the particle surface charge and the polymer charge density. Bridging polymers are characterized by high molecular weight and low charge densities. The flocs that are formed are large and voluminous. They have poor stability to shearing and will not reform to any great extent once sheared apart.
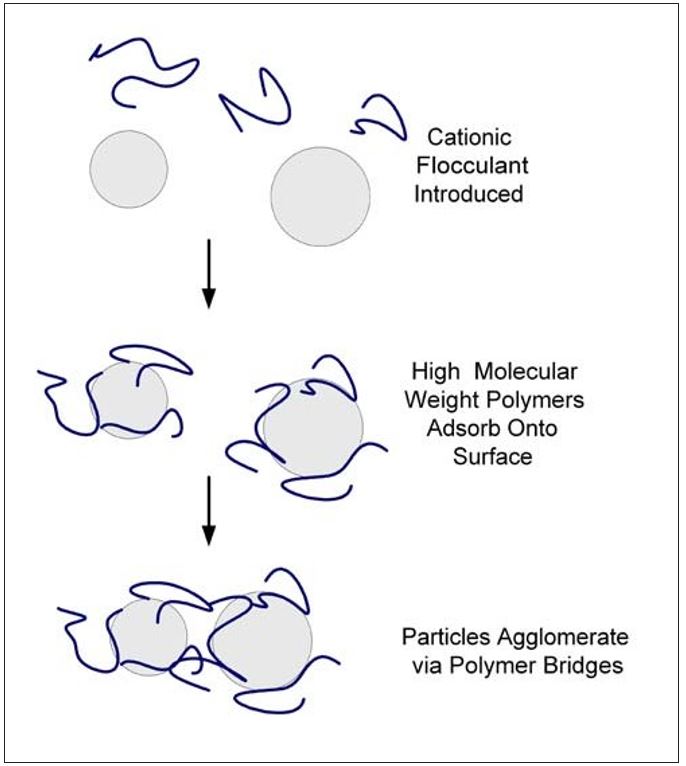 Figure 3 – Bridging Flocculation
Figure 3 – Bridging Flocculation
Of the complex flocculation mechanisms listed in Table 1, only the first two will be discussed here. As its name implies, the patch/bridge mechanism is a combination of the patch and bridge models already discussed. A classic example of patch/bridge complex flocculation is a dual polymer program consisting of a low molecular weight, high charge density cationic coagulant combined with a high molecular weight anionic flocculant (see Figure 4). As shown, the flocculation depends upon electrostatic attraction of the cationic patches to the anionic bridge. Important factors in the described interaction include the order of addition of the two polymers, as well as the time interval between their additions.
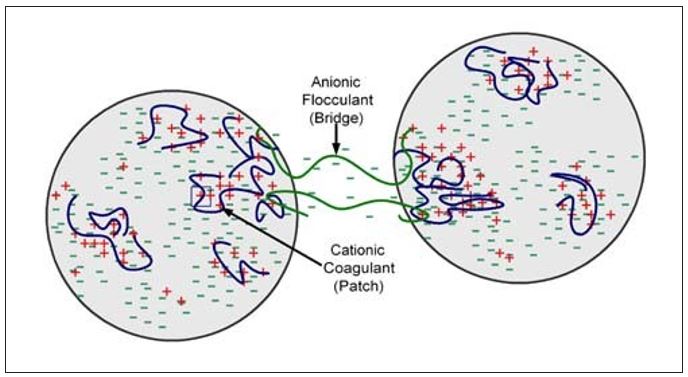 Figure 4 – Complex Flocculation – Patch/Bridge Mechanism
Figure 4 – Complex Flocculation – Patch/Bridge Mechanism
Finally, the complex flocculation mechanism utilizing microparticles (also called nanoparticles) is shown in Figure 5. The classic example of this type of program consists of a cationic flocculant (or starch) combined with an anionic microparticle. Many variants of microparticle programs exist that include cationic coagulants, anionic flocculants and a variety of other microparticles. In this mechanism, bridge formation is with the microparticle. Since the microparticle is the bridge, these flocs are at least partially reversible after shear exposure. Because reflocculation occurs on a microscale, improved dewatering, sheet formation and fine particle retention is often observed.
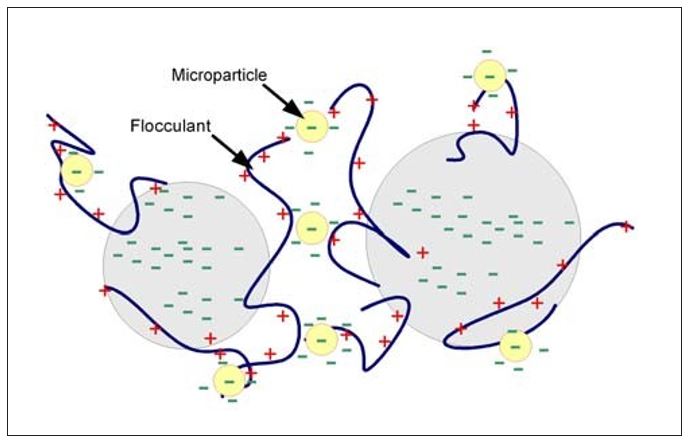 Figure 5 – Complex Flocculation – Microparticle Mechanism
Figure 5 – Complex Flocculation – Microparticle Mechanism
As already alluded to, retention aids can be classified into the broad categories of coagulants, flocculants and microparticles. Although inorganic coagulants (e.g. various aluminum species) are common in some segments of the paper industry, organic coagulants seem to be used almost exclusively in tissue and towel manufacture. Common chemistries include polyamines, polyethyleneimines and poly-(DADMAC)’s. These polymers have relatively low molecular weights and high cationic charge densities. Flocculants are typified by high molecular weight copolymers of acrylamide having cationic or anionic charges. A wide variety of charge densities are available depending on performance and regulatory needs. Common microparticle types include colloidal silica and bentonite.
Considerations for using a retention program on a tissue or towel machine will include the ability to deliver desired sheet properties, the efficient utilization of raw materials, as well as, improved machine efficiency/productivity. The focus in this paper will be the potential advantageous effects of retention on Yankee coating development and creping performance. However, the effect observed at the dry end is a cumulative one and is predicated on improvements made in the wet-end and press sections of the machine. To better understand the impact of retention aids on Yankee coating and creping, natural coating components and their deposition on the Yankee will first be reviewed.
2. Naturally-Occurring Coating Development on the Yankee Dryer
With ongoing development and use of synthetic Yankee dryer adhesives, releases and modifiers, there is a tendency to overlook the natural components of the tissue furnish that still contribute to coating build-up on the Yankee cylinder. Today, synthetic coating components can be designed for specific creping conditions, different grades and temperature and moisture environments at the Yankee. However in the not too distant past, natural coating components were the predominant means the tissue maker counted on to achieve a satisfactory coating on the dryer. These natural coating components may be listed as hemicelluloses, fines and fibrillar materials from the sheet, inorganics dissolved in the water and chemical additives used in the wet end of the machine.
Earlier research and review papers showed the importance of these natural coating components to coating development.4-7 Oliver5 outlined the potential mechanisms of natural adhesive layer build up from the wet end. These include (a) formation of an adhesive film by dissolved and dispersed hemicelluloses present in the process water as the water is evaporated on the Yankee dryer surface; (b) the transfer of small fibrillar material and fines from the tissue sheet to the tacky adhesive layer; and (c) the flow and transfer of hemicelluloses coating the outsides of the fibers onto the dryer surface under the influence of heat. Mechanisms (a) and (c), described for hemicelluloses, are also applicable to wet end additives such as strength resins. It is also possible to envision the fines described in mechanism (b) to be coated with hemicelluloses and/or wet-end additives that also contribute to coating development.
The deposition of soluble organic materials may be understood in terms of the “Dreshfield” effect.8, 9 Dreshfield’s thesis work studied the hot surface drying of paper and demonstrated how the water flow was from the inside of the web toward both surfaces of the sheet, where vaporization occurred. From both dye migration and moisture distribution data, he was able to show that a maximum moisture content occurred somewhere in a zone approximately 20 to 30% of the total sheet thickness, from the side of the sheet that was exposed to the open atmosphere. The lowest moisture content occurred at the closed surface of the sheet that was in contact with the heated metal (drying) surface. A second minimum in moisture content occurred at the open surface. These findings are summarized in conceptual form for the case of Yankee drying in Figure 6. Assuming an approximately uniform z-directional moisture distribution in the web as it leaves the pressure roll nip, Figure 6 shows the moisture distribution in the sheet going from the Yankee surface to the air side.
As the water is vaporized at the sheet surfaces, soluble organic and inorganic as well as small colloidal materials in the water are left behind. Similar to Dreshfield’s findings with a soluble dye, Figure 7 conceptualizes the concentration of soluble materials on both the Yankee and open (air) sides of the sheet. The effect is the inverse of that shown in Figure 6. Another way to visualize this process is provided by the diagram in Figure 8. Here the soluble materials are represented by the red dots, and the concentration from the water phase to the surfaces of the sheet is shown. The addition of these materials into the boundary layer of the Yankee coating is further depicted in Figure 9. It now becomes evident, as most tissue makers will recognize, why increased Yankee drying (vs. hood drying) can lead to increased (natural) coating on the dryer. Total Yankee drying of the sheet is identical to Dreshfield’s original experiments, leading to greater vaporization and deposition of soluble materials at the closed surface. Increased drying with the hoods will lead to more water vaporization from the air side of the sheet and less natural coating development at the Yankee surface.
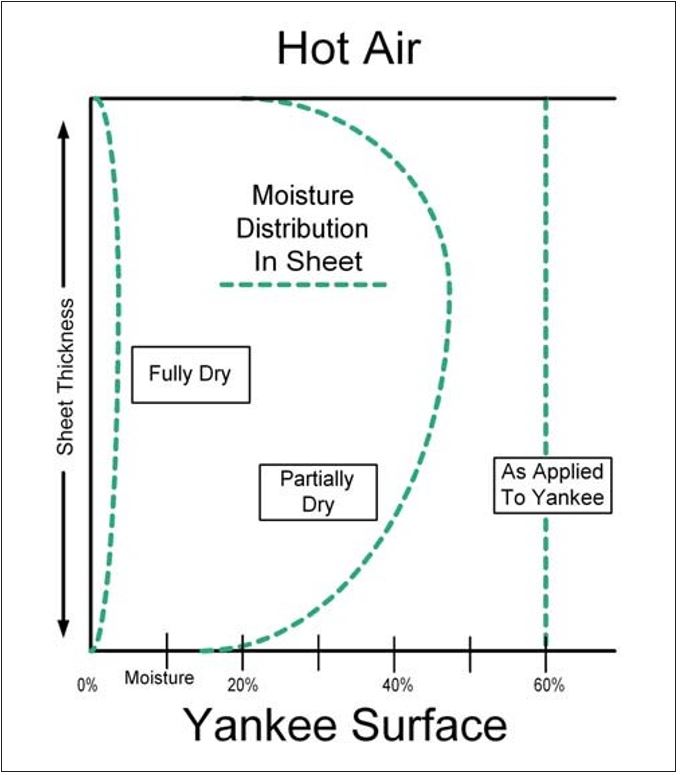 Figure 6 – Moisture distribution within sheet during drying (per Dreshfield8)
Figure 6 – Moisture distribution within sheet during drying (per Dreshfield8)
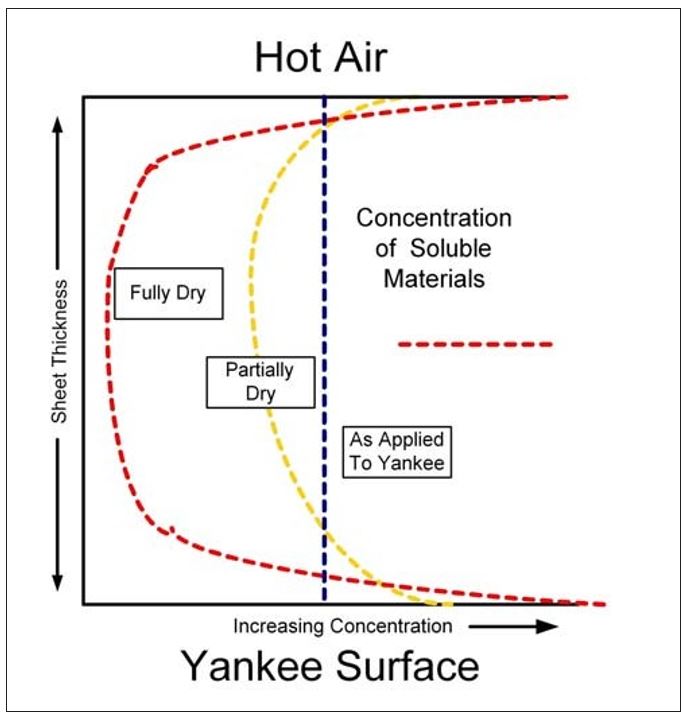 Figure 7 – Distribution of soluble materials in the sheet during drying
Figure 7 – Distribution of soluble materials in the sheet during drying
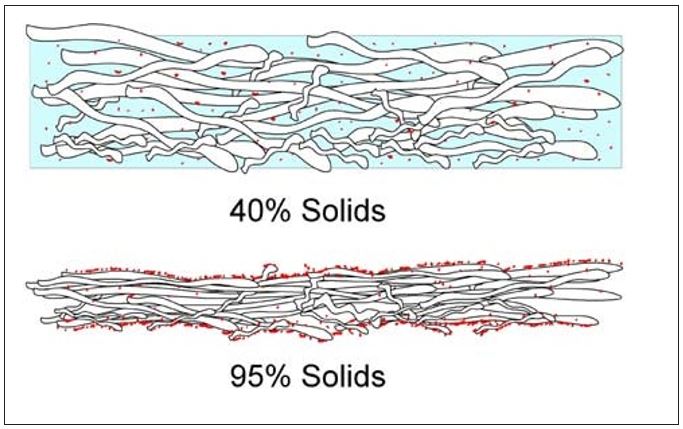 Figure 8 – Movement of soluble materials to surfaces of the sheet during drying
Figure 8 – Movement of soluble materials to surfaces of the sheet during drying
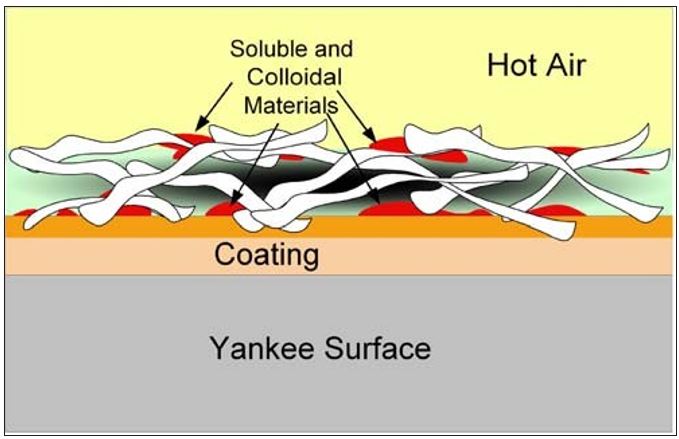 Figure 9 – Transfer of insoluble materials from sheet surface
Figure 9 – Transfer of insoluble materials from sheet surface
into the boundary layer of the Yankee coating
Inorganic materials are known to play a role in coating development. In particular, it is widely accepted that water hardness, within a certain range (Oliver5 states 90-125 mg calcium carbonate/liter), is beneficial for coating development. The inverse solubility of CaCO3 enhances the deposition of this material from solution in the early stages of drying and its contribution to coating development. Schiel7 provides a conceptual picture of the inorganic materials as additional anchor points for organic coating development. This inorganic deposition helps stabilize the coating and in effect makes it more durable. However, too much inorganic content in the coating is not a good thing, since at some point adhesion and coating durability will be negatively impacted.
Wet-end variables such as furnish composition, refining level, pH and functional additives can all play a role in natural coating development. These variables influence the amount of hemicellulose, CaCO3 and wet end additives available to contribute to natural coating. For example, the contribution of wet strength resins to coating development is widely known. Common wet strength resins and most commercial Yankee adhesives belong to the same general class of polymers. However, one important difference between the poly(aminoamide)-epichlorohydrin (PAE) resins used for wet strength and those used for creping adhesives is the level of crosslinking functional groups present. Wet strength resins have a much higher potential for further crosslinking. Hence, it is relatively easy to understand that wet strength resins used in the wet end of the paper machine can deposit on the dryer through the mechanisms previously outlined and contribute to and affect the properties of the coating. The active crosslinking of the wet strength resins in the Yankee coating during drying will produce a coating that may be described as harder and more durable, but with less adhesion. Figure 10 illustrates the increase in durability found for four different PAE resins. Each of the PAE resins had a different level of active crosslinking sites, with PAE 1 having the highest number and PAE 4 having essentially none. The laboratory measurements show solubility decreased with the number of active crosslinking sites.
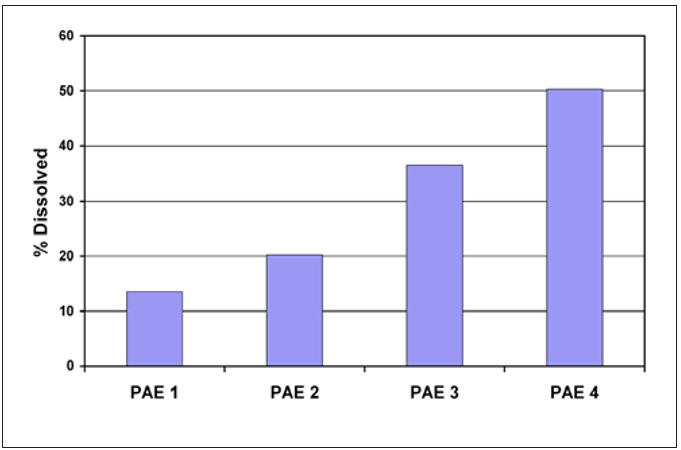 Figure 10 – Differences in PAE durability (as indicated by film solubility)
Figure 10 – Differences in PAE durability (as indicated by film solubility)
Since retention aids will increase the amount of fines and colloidal materials in the sheet, they will obviously enhance natural coating mechanism (b) described above. These fines and colloids can transfer from the sheet and contribute to coating build-up on the Yankee. It is also possible to bring more dissolved hemicellulose out of the water phase, in the form of complexes with the retention aid, and precipitated onto the fiber surfaces, to the dry end of the tissue/ towel machine. These can then flow, during drying, from the fiber surfaces into the coating as described by natural coating mechanism (c). By retaining more functional additives (e.g. wet strength) in the sheet, retention aids can also make more of these components available for contribution to the coating at the dry end. These changes will be evident by increased thickness of the coating, as well as potential differences in the coating adhesion, softness and durability. It is important to recognize that changes to synthetic materials sprayed on the Yankee dryer can be made in order to take advantage of the increased coating, while maintaining desired coating characteristics.
B. COMMERCIAL RESULTS FROM RETENTION PROGRAMS
With the scientific background established above, results will now be presented to demonstrate the impacts of retention aid programs on tissue/towel machine dry end performance. First, we present a survey of retention aid impacts on a study set of 10 machines. Next, two specific case studies are provided to illustrate improvements in wet-end process variability and felt performance and the related benefits to Yankee coating and creping.
1. Survey of Retention Aid Impacts
Table 2 summarizes data collected from retention aid programs run on 10 different tissue/towel machines. Although the table is just a subset of the total number of machines that have used retention aids, the data is informative and appears directionally accurate. Included in the survey are different machine types, furnishes, and retention aid programs. The retention programs ranged from coagulant only programs, to flocculant programs, to advanced microparticle programs.
First Pass Retention (FPR): All 10 machines noted an increase in FPR ranging from 5 to 20 percentage points. The magnitude of the increase depended upon the specific retention chemistries applied, the addition rate of those chemistries, and differences in process variables. The effectiveness of the retention programs was related to the following items:
•Fiber selection
•Baseline wet-end chemical addition
•Water system closure
•Level of refining
The primary benefit associated with the increase in FPR was the potential to increase the fiber to paper ratio, or process yield. Additionally, the increased fiber retained with the sheet resulted in a lower solids load to the white water process clarifier (DAF) and a reduction in waste from this unit. Yield improvements varied between 1-5% depending upon whether the process utilized fiber recovery screens or recycled a portion of the DAF solids.
Yankee Adhesive Usage:
Six of the 10 machines observed an increase in Yankee coating thickness or adhesion. This benefit was captured by reducing adhesive add-on rates on five out of the six machines. One machine utilized the increase in adhesion to improve the creped sheet structure. The magnitude of the impact on coating corresponded to the increase in FPR:
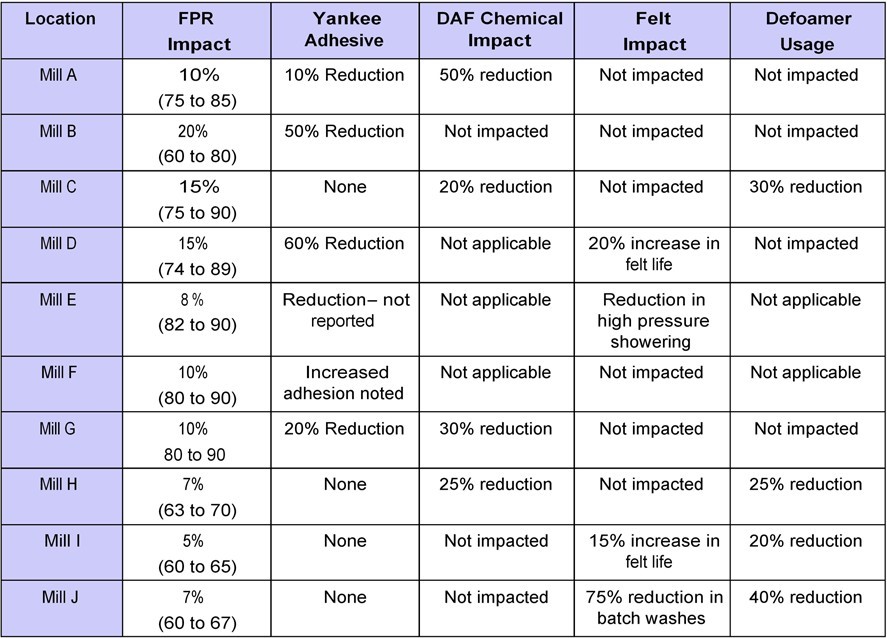 Table 2 – Survey results from 10 machines running retention aids
Table 2 – Survey results from 10 machines running retention aids
•Five out of six machines that reported increases in FPR greater than 10 percentage points, saw an increase in coating thickness or adhesion.
•The two machines that reported the highest increase in FPR saw the largest increase in coating thickness or adhesion.
•Only one out of four machines that reported increases in FPR less than 10 percentage points saw an increase in coating thickness or adhesion.
The data illustrate that increases in FPR translated to increases in the amount of natural coating transferred to the Yankee dryer by the mechanisms described previously.
DAF Chemical Reduction:
A typical machine water system sends a portion of the white water from the flume or silo to a supply tank where it is combined with various other process streams. This mixture is then pumped to a DAF or clarifier, which combines mechanical action and chemical treatment to separate the majority of the influent solids content into what is commonly called “sludge.” The clarified water is returned to the process for reuse. The amount of chemicals needed to treat the influent is primarily dependent upon the influent solids content.
By increasing retention, the percentage of solids equilibrium in the white water system is significantly reduced. This ultimately lowers the solids load to the DAF and the amount of chemical treatment needed for efficient unit operation.
Three of the machines listed in Table 2 did not utilize chemical treatment in their clarification operation, or sent the dirty water to a common mill DAF. Four out of seven machines that utilized an on-machine DAF or clarifier reported a reduction in chemical treatment. The magnitude of the impact depended upon:
•Use of fiber recovery screens before the DAF
•Mechanical design of the DAF
•Baseline DAF efficiency targets
While three of the seven applicable machines did not quantify a reduction in DAF chemical usage, they likely benefited from improved DAF efficiency or screen efficiencies that were simply not recorded before or during the data collection period.
Felt Performance Impact:
Four out of the 10 machines reported an improvement in felt performance as detailed below:
•Mill “D” and Mill “I” noted an increase in their felt life. This resulted from a reduction in high-pressure shower set points that ultimately reduced the wear rate of the fabric.
•Mill “J” saw a 75% reduction in the number of batch washes needed to treat the felt for both fines and wet strength contamination that resulted in increased production.
•Mill “E” observed a reduction in high-pressure shower set points, although this has not translated into improved felt life.
A more in-depth discussion of the impact of retention aids on felt performance and the resulting benefits to Yankee coating is provided in the specific case study, “Improvement in Felt Performance,” below.
Defoamer Usage:
Two of the machines in this study did not apply a defoamer program to control white water entrained air and foaming levels. Four of the remaining eight machines saw significant reductions in defoamer usage ranging from 20-40%. This occurred as the retention aids reduced the amount of unretained colloids and fines in the white water loop. The following operating conditions helped determine the potential of the retention program to reduce defoamer application rates:
•Defoamer control strategies varied from judging usage rates based on foam or entrained air to base loading a set amount at all times and not changing the set point without a compelling reason. It is possible that foaming was impacted during the trials, but defoamer addition rates were not adjusted to compensate for the change.
•Addition strategies can impact the amount of unretained wet strength resin present that can lead to white water foaming. Three out of the four machines that reported a reduction in defoamer use were also using wet strength.
•The use of anionic wet strength promotion chemistries varied in this study. The use of a wet strength promoter can reduce the amount of unretained wet strength and subsequent foaming tendencies of the white water.
A decrease in defoamer use is important not just from a savings standpoint, but also because many defoamer formulations contain hydrophobic components that will contribute to release and hinder coating development on the Yankee. If defoamer addition varies due to unstable wet end conditions, then an additional, and sometimes unrecognized, source of variation in coating performance can occur.
2. Improvement in Wet-End Process Variability.
The implementation of a retention aid program, coupled with customized control strategies, can reduce wet-end and subsequent Yankee coating variations. By targeting a FPR, or in this case, a white water turbidity, and controlling it through changes in retention aid addition rates, the stabilized wet-end will result in a more uniform creping operation.
There are several ways to monitor FPR:
•Online retention monitoring systems are available, but they are capital and maintenance intensive.
•Manual measurement of FPR is time consuming and samples cannot be processed quickly enough for real time monitoring and control.
•In many cases, manual white water turbidity measurements are representative of the FPR and the data takes only seconds to obtain.
•Online white water turbidity monitoring systems can be incorporated into the process control system to adjust retention aid addition rates in order to maintain a turbidity set point.
Mill K provides a specific example of improved wet-end process stability. This mill experienced periodic loss of process reliability due to uncontrolled swings in retention and solids loading in the white water loop. Changes to the refiner or furnish blend often resulted in streaks developing in both the coating and on the fabric. Pick-outs and off-quality finished product were causing significant losses in production. Nalco and the customer worked together to implement a flocculant-based retention program along with a strategy to control white water turbidity to a target of 125 NTU’s. The turbidity was measured once per shift and the retention aid dose was adjusted to stay within statistically set control limits. Figure 11 illustrates how the implementation of this strategy both cleaned up the white water system and reduced the turbidity variation. The periodic machine upsets experienced previously were significantly reduced. These appeared to be a function of the wet-end variation seen prior to implementation of the retention program.
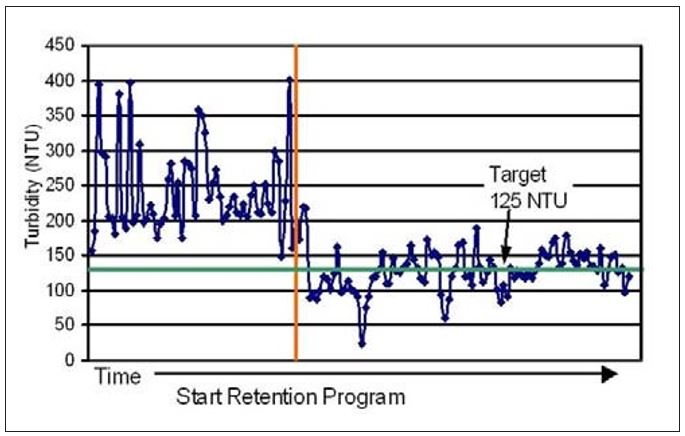 Figure 11 – Changes in white water turbidity upon application
Figure 11 – Changes in white water turbidity upon application
of a retention program at Mill K
The decreased white water turbidity and lower variation corresponded to improved FPR and decreased variation in these values as well (see Figure 12). These results, therefore, support the approach of using whitewater turbidity as a means to monitor and control retention programs. The decreased variability of both FPR and whitewater turbidity were good indicators of a reduced MD variability in the amount of colloidal and fines material carried with the sheet to the Yankee. Since some of these materials are ultimately transferred to the coating, the reduced variability contributes to more stable and consistent coating development and coating properties. With a consistent amount of natural coating contributing to film thickness and adhesion, a more stable creping operation ultimately results.
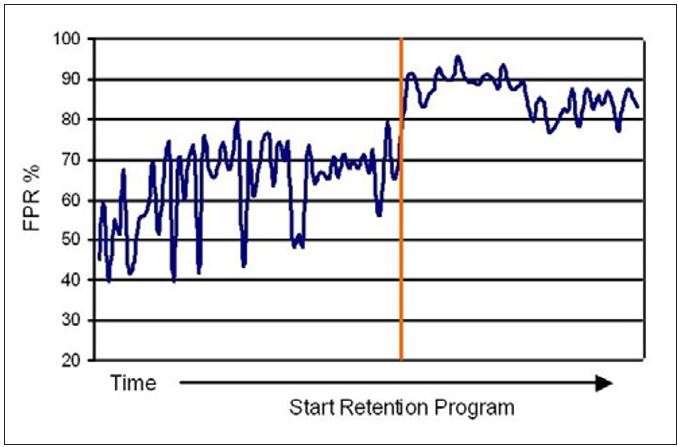 Figure 12 – Changes in First Pass Retention (FPR) upon application of a retention program at Mill K
Figure 12 – Changes in First Pass Retention (FPR) upon application of a retention program at Mill K
Improved DAF efficiency indirectly impacts coating performance through two primary mechanisms:
•Clarified water quality impacts both wire and felt showering. Poor water quality due to DAF upsets is usually first noticed when machine roll covers begin to experience fiber build-up. The poor water quality will also reduce felt void volume and Suction Pressure Roll (SPR) web solids, thus impacting the coating development at the SPR / Yankee interface.
•Clarified water is often used for consistency control or pulper dilution, which impacts the entire process. An increase in fines in the clarified water will reduce chemical efficiencies throughout the process and likely reduce FPR.
In Mill K the increased FPR, along with lower variation, resulted in a consistently reduced solids load to the DAF unit. A significant improvement in DAF efficiency with reduced variation in the clarified water quality was observed (see Figure 13).
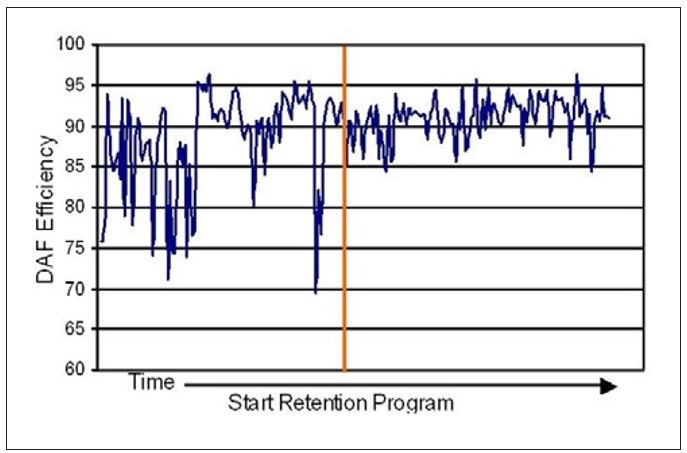 Figure 13 – Changes in DAF efficiency upon application of a retention program at Mill K
Figure 13 – Changes in DAF efficiency upon application of a retention program at Mill K
3. Improvement in Felt Performance.
Tissue makers take great care to reduce CD variation across the felt by utilizing the following common mechanical cleaning elements:
•Oscillating high pressure needle showers
•Moderate pressure chisel fan showers
•Low pressure flooded nip showers
•Uhle box optimizations
These mechanical cleaning elements are the key to maintaining felt performance. However, additional chemical cleaning applications are also available and are listed below:
•Sheet side passivation to prevent contaminant deposition
•Batch cleaning on shutdowns to dissolve or remove contaminants
•Continuous cleaning to assist in mechanical removal of contaminants
The mechanical strategies, while effective, can lead to premature felt wear and excessive water carryover that reduces drying capacity. Chemical cleaning adds cost and batch cleanings on the down result in lost production. In an effort to identify the root cause of felt contamination, Nalco Europe B. V. (Leiden, Netherlands) accumulated data associated with felt analyses from tissue machines.10 Their analysis of 40 felts showed the major source of contamination was due to non-extractable solids. Furthermore, fiber fines were the largest contributor to the nonextractable fraction of the contaminants. Fixing the fines to the sheet by utilizing a retention program, instead of having them migrate into the felt, would seem to be a logical way to keep the felt clean.
As previously shown in Table 2, four out of 10 machines documented an improvement felt performance. More detailed information was obtained from a recent trial where the primary objective was to reduce felt filling by addition of a coagulant to improve fine particle retention. The trial ran across the entire life of a felt to compare baseline felt life data against that obtained during the trial period. The following primary variables were monitored:
•White water turbidity
•Suction pressure roll vacuum
•Baseline and trial felt contaminant analysis
•Production
•Felt life
Table 3 illustrates the results from this effort and confirms substantial benefits accrued due to the use of a retention aid. The significant reduction in white water turbidity verified the selected coagulant chemistry was active in the system. Although the FPR only improved about five percentage points, the white water turbidity was reduced by 75%, indicating good fine particle retention. During the trial period, the suction pressure roll vacuum remained lower than historical trend lines, indicating the felt was not filling as fast. This reduced batch on-the-down cleanings, a practice machine personnel traditionally utilized to keep the felt open. Additionally, reductions in high pressure shower set points reduced felt wear, and increased felt life.
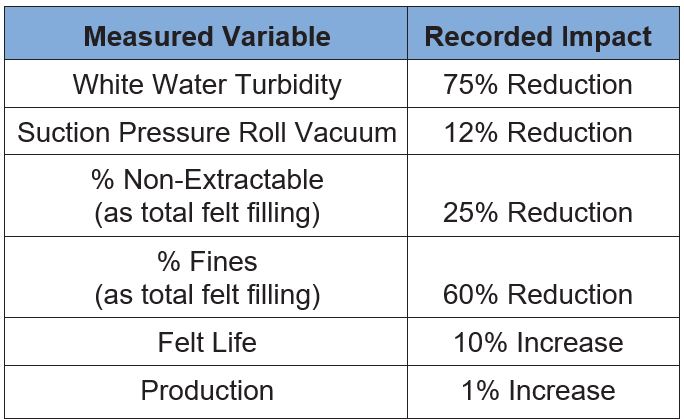 Table 3 – Mill L Felt Performance Data Summary
Table 3 – Mill L Felt Performance Data Summary
Direct confirmation that the retention approach had worked was found from the felt analysis completed after the trial period was over. Total non-extractables were reduced by 25% compared to baseline averages. This improvement seems to be driven by the retention program’s ability to keep the fines with the sheet as percentage fines filling was reduced by 60%. Furthermore, the felt life improvement was sustainable through additional felt cycles as shown in Figure 14. Overall, the reduction in batch washes combined with the improved felt life has led to a 1% improvement in annual production.
Optimal dewatering and lamination of the fibrous web within the Yankee-SPR nip is directly related to sheet adhesion at the creping doctor blade and, thus, the creping transformation. Since the felt is a functional element within the nip, variability of felt performance must be reduced in order to reduce variation of the creping transformation. Moisture variation exiting the nip will be directly related to felt permeability, that is, the more resistance to flow in the felt, the higher the moisture in the sheet exiting the nip. Archer et al.11 previously described the importance of the felt in nip dewatering dynamics. Excess water in the nip will dissolve, or overly soften, the coating leading to streaks and non-uniform creping. The beneficial effect of using a retention aid to reduce variation and extend the optimal performance lifetime of the felt is an effective tool for tissue makers to consider.
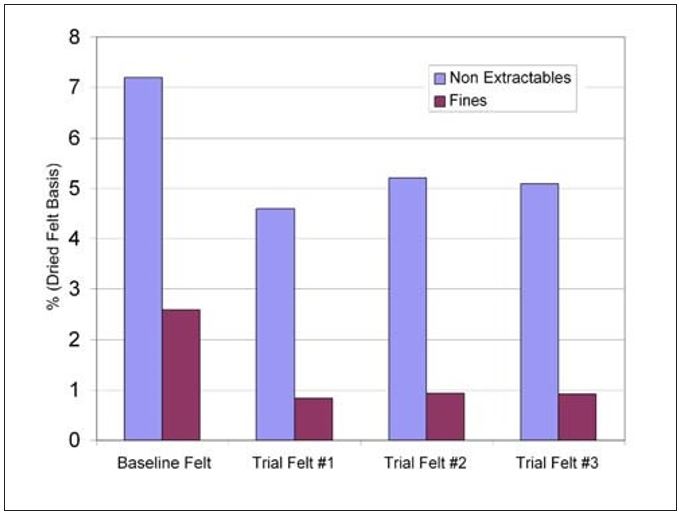 Figure 14 – Mill L felt analysis results for nonextractables and fines contaminants
Figure 14 – Mill L felt analysis results for nonextractables and fines contaminants
C. CONCLUSIONS
Data collected from a wide variety of tissue/towel machines was used to show the impact of retention aids on Yankee coating. The effect is cumulative through the machine, and examples were provided of the contributions from improved wet-end stability, improved felt performance, as well as increased levels of coating on the dryer that can improve dry end tissue/towel machine operations. Increases in coating thickness and adhesion roughly correlated to the magnitude of FPR improvement upon implementation of a retention program. Two cases were documented where 15-20 percentage point improvements in FPR led to 50-60% reductions in Yankee adhesive use. The improvement in coating thickness can be explained by increased levels of natural coating components contributing to the coating. Even small improvements in FPR can lead to large improvements in fine particle retention. The improved small particle retention can evidence itself in improved felt cleanliness, DAF efficiency and reduced defoamer use. All of these improvements help coating development in the pressure roll nip and ultimately lead to better creping.
REFERENCES
1. Scott, W. E., “Fines Management and Control in Wet-End Chemistry,” Tappi J. 69, no. 11, 30-34 (1986).
2. Eklund, D. and Lindstrom, T., Chapter 7, “Retention and Dewatering,” in Paper Chemistry, An Introduction. Grankulla, Finland: DT Paper Science Publications, pp. 145-191 (1991).
3. Kim, Y. H. Coagulants and Flocculants, Theory and Practice. Littleton, CO: Tall Oaks Publishing, Inc., 1995.
4. Fuxelius, F. K., “Adhesion of Paper Web to Glazing Cylinders During Dry Creping,” Sven. Papperstidn. 70, no. 5, 164-168 (1967).
5. Oliver, J. F., “Dry-Creping of Tissue Paper – A Review of Basic Factors,” Tappi J. 63, no. 12, 91-95 (1980).
6. Nordman, L. and Uggla, R. “Adhesion Between Fibre Webs and Metal Surfaces During Drying,” in Fiber-Water Interactions in Papermaking. Transactions of the BP BIF Symp. Oxford, pp. 459-75 (1977).
7. Schiel, C., “Economic and Technical Aspects in the Production of Lightweight Sanitary Tissue – Between Headbox and Yankee Dryer,” Das Papier 38, no. 9, 417-429 (1984).
8. Dreshfield, A. C. A Study of Transverse Moisture Distribution and Movement During Hot-Surface Drying of Paper. Ph.D. Thesis, The Institute of Paper Chemistry (1956).
9. Dreshfield, A. C. and Han, S. T., “The Drying of Paper,” Tappi J. 39, no. 7, 449-455 (1956).
10. da Silva Santos, C., “Optimizing Pick-up Felt Conditioning on the Run Improves Tissue Machine Efficiency,” Tissue World (OctoberNovember, 2003).
11. Archer, S., Furman, G. S. and Daily, W., “Creping Optimization, Look One Step Back,” Tissue World Americas Conference, Miami, FL (March 2006).
ACKNOWLEDGEMENTS
The authors would like to acknowledge their colleagues at Nalco for providing the data for the supporting case studies.


Comments are closed.